Study Background & Design
This observational study explores how changes in sleep and daily activity affect mood in people living with bipolar disorder.
We aim to develop a risk algorithm that detects early warning signs of mood episodes. After this project is complete, this algorithm could be embedded into an actigraph—a research-grade activity tracker like a Fitbit—to help people better manage their bipolar disorder.
The study is funded by the Wellcome Trust and conducted by a team of researchers, clinicians, and lived experience collaborators from Swinburne University, University of Otago, Institute of Technology Hyderabad, Newcastle University, and the University Medical Center Hamburg-Eppendorf.
- 1 x Online screening survey and baseline interview (2–3 hours total)
- 6 x Follow-up online surveys and interviews (1–1.5 hours total)
- 3 x 14-day periods of wearing activity-tracking devices (MiEye and actigraph) with daily sleep diaries and brief surveys at the end of each period (5–10 minutes per day)
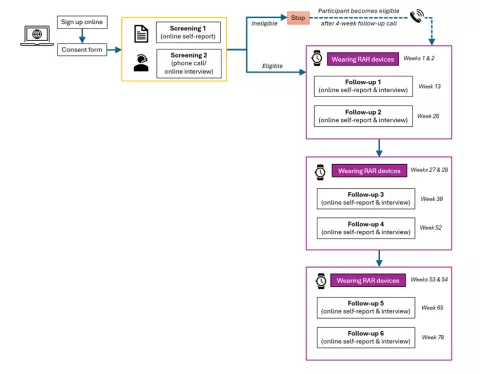
A flowchart on the website outlines your full participation timeline.
Study Data
We collect data via:
- Wearable devices that track light exposure and activity
- Short surveys about your mood and daily routines
- Online video interviews at key points in the study
All data is stored securely on Swinburne University servers. Your information is deidentified and linked to a Study ID, not your name.
Results will be shared once the study is published. This may take several years.
During each two-week "burst" period, you'll complete brief daily sleep diaries (sent at 9 AM AEST via email). You’ll have until midnight to complete them, with two automated reminders. This data is essential to understanding how sleep may influence relapse risk.
Yes. Survey timing will vary based on your time zone. We’ll confirm how this affects you and help you plan accordingly.
Study Devices
- Actigraph (GENEActiv): A waterproof wrist-worn device that tracks movement, light, temperature, and sleep.
- MiEye: A small, non-waterproof light sensor that monitors your daytime light exposure.
To study whether changes in sleep and light exposure are linked to relapse risk in bipolar disorder.
- MiEye: 7 days during the day (remove at night, in water, or during contact sports).
- Actigraph: 14 days, worn 24/7 (including in water).
Contact the research team ASAP. If after hours, leave a message or email. We'll send a replacement and help you resume the study.
Let us know in advance. We'll work with you to reschedule the burst period.
That’s okay—just keep us informed so we can interpret your data correctly.
Participation & Eligibility
No. Your involvement in the study will not interfere with your day-to-day life at all, nor will your treatment be changed or impacted.
Those who are:
- Currently diagnosis of bipolar disorder I or II
- Aged 18 – 65 years old
- Not currently psychotic or suicidal
- Not diagnosed with schizoaffective disorder
- Able to understand the plain language statement and able to provide consent
- Able to provide photo ID as proof of identity (to combat fraud)
- Able to demonstrate an understanding of written and spoken English
- Not experiencing side effects from current medication regime
- Not suffering from any physical condition or experience any difficulties that may impact their ability to participate in the current study
- Not living a lifestyle that would impact their ability to participate effectively in the current study (e.g. caring for infants, irregular shifts including night shifts)
Read and sign the PICF and fill in the first screening questionnaire
Participation is voluntary. You can withdraw at any time if you decide the study isn’t right for you.
We will assess your mood symptoms, but treatment decisions are up to you and your healthcare providers. Our team will only intervene if we have serious concerns about your safety or that of others during an assessment. In that case, we’ll pause the interview and connect you with a senior study clinician for support.
At the start, you will provide details of your treating practitioner. If this changes during the study, please notify us so we can update our records.
We will keep a record of a contact person responsible for your care in case we have concerns about your mental health or safety. This study is observational only and doesn’t affect your treatment.
Treatment includes any intervention by a medical professional aimed at maintaining stable mood, such as therapy or prescribed medication.
Any treatment changes are managed between you and your medical team. We’ll record those updates during follow-up interviews.
Postage & Remote Participation
No travel is required (except going to your local post office to post the study devices back). All interviews are conducted remotely via Microsoft Teams.
Let us know your plans, and we’ll adjust study appointments to fit your schedule.
Devices are sent via Australia Post Express with tracking. Your monitoring period starts the morning after delivery.
A prepaid return envelope with instructions will be included in your package.
Interviews
- Baseline interview: Up to 3 hours plus brief surveys (5–15 minutes)
- Follow-ups (6 total): 45–75 minutes plus short surveys (5–10 minutes)
Seven in total: one baseline and six follow-ups.
Yes, to ensure accurate data. Recordings are securely stored and accessed only by the research team.
Individual results are not shared. De-identified group data will be available once the study is published.
Yes, your treating practitioner will receive a letter confirming your participation in a research study at Swinburne University of Technology.
Reimbursement
Payments are made via Giftpay e-gift cards, typically within 48 hours after completing each study component.
- Screening + Baseline: $112.50
- Six follow-up interviews: $90 each ($540 total)
- Wearing devices (3 periods): $150 each ($450 total)
- Completion bonus: $100
- Plus, entry into a $1000 prize draw (10 prizes available)
Maximum total: $1,102.50 (excluding prize draw winnings)
Support During the Study
You can skip questions or pause the interview at any time. For treatment or urgent care, please contact your healthcare provider or use crisis resources listed in the Participant Information and Consent Form (PICF).
Our team can also arrange immediate support through the lead study clinician if needed.
Additional Support Resources
For Australian participants:
- Lifeline: 13 11 14 (24/7 crisis support)
- Beyond Blue: 24/7 mental health support
- Black Dog Institute: Information and self-assessments
- SANE Australia: Resources for bipolar disorder
- Health Direct: General health information
For New Zealand participants:
- Lifeline Helpline NZ: 24/7 phone/text support
- Health New Zealand Te Whatu Ora: Mental health crisis support and local resources
